Research into alternatives to petroleum oils began long before Nazi Germany invaded Poland in 1939. But not until WWII choked off Germany’s crude-oil supplies and dramatically revealed petroleum oil’s failings on the front lines did a clear incentive to develop synthetic oil emerge. Coincidentally, as Germany’s soldiers went backward on the battlefield, its scientists drove synthetic-oil technology forward in the laboratory. More than two decades later, a fighter pilot from Duluth, Minn., would take up the mantle and bring synthetic oil to the automotive world. Here’s the story of how a technology forged in the world’s bloodiest conflict arrived in the vehicles we drive today.
Much must have weighed heavily on the minds of German and Russian soldiers as Germany’s 6th Army besieged Stalingrad, Russia on Aug. 23, 1942. Hitler had targeted the industrial city since it produced artillery and served as an important shipping route to the country’s eastern regions. Perhaps as importantly, he prized the city because it bore the name of his adversary – Joseph Stalin.
Maybe the Germans were thinking about their defeat earlier that winter in a failed attempt to take Moscow. Maybe the Russians had in mind Hitler’s proclamation that, upon taking Stalingrad, he’d have all the city’s men killed and its women deported. Whatever the case, surely none of the soldiers or civilians had petroleum oil and its propensity to solidify in the cold on their minds.
However, as the fighting wore on through the winter, petroleum oil’s shortcomings emerged as one of several reasons the Germans lost the Battle of Stalingrad. Despite early gains by Germany throughout the late summer and fall, the Russians refused to surrender. By late November, they’d trapped what was left of Germany’s 6th Army in a defensive ring around the city. Then Russia’s brutal winter set in. Hitler refused to surrender even as his soldiers slowly starved and ran out of provisions. Adding to the catastrophe, the army’s tanks, aircraft and other military vehicles refused to start due to petroleum oil solidifying in the bitter cold.
The battle ended in February 1943 as Hitler’s first publicly acknowledged failure of the war. It signaled a major defeat for the Axis powers. And it provided dramatic evidence of the inadequacy of petroleum motor oil to perform in temperature extremes.
The Birth Of Synthetic Oil
Decades before the Battle of Stalingrad, scientists had been searching for an alternative to petroleum oil. In fact, French chemist Charles Friedel and his American collaborator, James Mason Crafts, first produced synthetic hydrocarbon oils in 1877. In 1913, German scientist Friedrich Bergius developed a hydrogenation process for producing synthetic oil from coal dust. Twelve years later, his countrymen, Franz Fisher and Hans Tropsch, developed a process for converting a mixture of carbon monoxide and hydrogen into liquid hydrocarbons. In America, meanwhile, Standard Oil Company of Indiana tried to commercialize synthetic oil in 1929, but lack of demand doomed the attempt. That didn’t stop Standard Oil researcher F.W Sullivan from publishing a paper in 1931 that disclosed a process for the polymerization of olefins to form liquid products. At about the same time, German chemist Hermann Zorn independently discovered the same process. Their discoveries laid the groundwork for the eventual widespread use of synthetic oil.
For the time being, however, conventional petroleum oil remained the dominant technology. The distillation process used to make conventional lubricants hasn’t changed much since then. Formulators start with crude oil, which contains wax and a mishmash of elements, such as sulfur, nitrogen, oxygen and various metals. Many materials inherent to crude oil must be removed through refinement to increase the oil’s usability. Refiners do this by applying heat, pressure and other catalysts to separate crude oil into different groups, called fractions. Further processing results in many of the products we use today, such as kerosene, gasoline, diesel fuel and lubricating oils used to make conventional motor oil.
The Limitations Of Distillation
As soldiers on the front lines discovered, however, conventional lubricants have inherent limitations. Distillation cannot completely remove impurities detrimental to lubrication, such as waxes that solidify in the cold and prevent engines from starting. Nor can it remove the lighter, unstable molecules that evaporate due to high heat. The extreme conditions of warfare exposed the limitations of conventional oil. It became obvious the world needed a better oil.
Necessity Drives Synthetics Forward
Synthetic lubricants were the answer. Unlike their conventional counterparts, synthetic oils are “built,” not distilled. This means formulators start with individual molecules, typically ethylene if formulating polyalphaolefin (PAO)-based synthetic oil, and build the lubricant from the ground up in the laboratory.
To illustrate, think of crude oil like a pile of LEGO* blocks haphazardly connected to form various shapes of different sizes (see inset). Each block represents a different molecule, including elements such as carbon, sulfur, nitrogen or oxygen. Distillation separates the blocks into piles based on size. Larger blocks form a pile, medium blocks form another pile and so on. Each pile is analogous to a crude-oil fraction. The fraction containing smaller, lighter molecules is used to make products like kerosene and gasoline. Larger molecules become tar. Medium molecules become products that include base oils.
Distillation cannot prevent irregular molecules or molecules unsuited for lubrication from contaminating the fraction intended for lubricating oils, reducing the finished product’s performance.
The process used to make synthetic oil solves this problem by removing contaminants. Formulators start with a crude-oil fraction, or a pile of LEGO blocks to continue the analogy. They use different chemical processes to “crack” the blocks into individual LEGO bricks, deconstructing each larger molecule into its constituent parts. They’re left with different molecules, like LEGO bricks spread out on a table.
They select only the pure, uniform materials best suited for lubricating an engine, which is typically ethylene when manufacturing synthetic lubricants. Using organic synthesis, chemists use ethylene to build larger molecules, called alphaolefins. Then they use alphaolefins to build polyalphaolephins (PAO). “Poly” simply means “many.” The final product is a PAO synthetic base oil used to make synthetic motor oil.
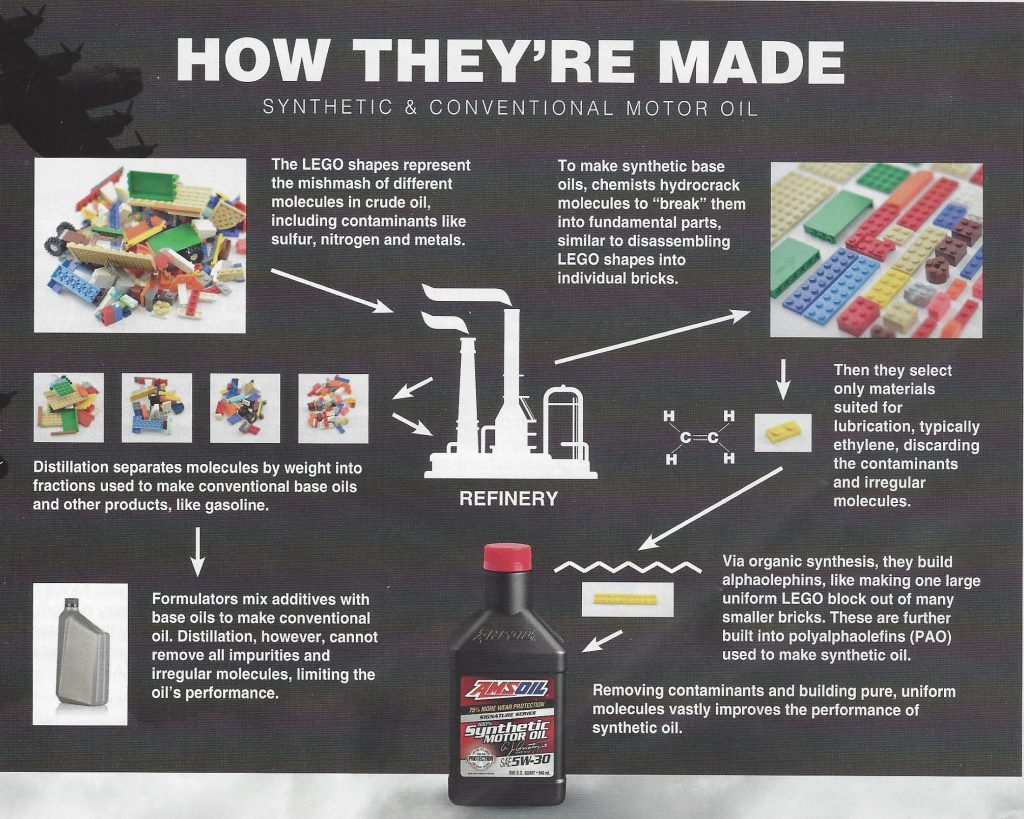
By building the finished product from only pure, uniform molecules, synthetic oils remain fluid in sub-zero cold for easier starts and better startup protection, resist evaporation in extreme heat, provide better wear protection and last longer. Given their superiority, it’s easy to see why synthetics had been gaining popularity even before the war.
But the tipping point didn’t come until the war choked off supplies of petroleum oil to several countries, notably Germany, France and Japan. The Stalingrad disaster coupled with lack of crude oil forced Nazi Germany to undertake an intense effort to find alternatives to petroleum oil. Zorn and his colleagues investigated a wide range of synthetic base-fluid chemistries, many originating from coal and other biobased sources. Germany evaluated more than 3,500 synthetic esters between 1938 and 1944. Their superior performance made them the focus of Germany’s synthetic lubricant technology during the closing years of the war.
By 1968, AI Amatuzio was commercially selling synthetic motor oil for automotive use.
In America, meanwhile, W.A. Zinsman led a more limited research program into synthetics at the Naval Research Laboratory between 1942 and 1945. The result was the development of the first diester synthetic base oils.
Synthetics Take Flight
The increased performance demands of aircraft engines helped drive development of synthetic oil during the war. But the emergence of aviation gas turbine engines at the end of WWII and during the post-war era brought synthetics to the forefront. Conventional oils were incapable of providing the extreme-temperature protection required of jet aircraft. Only synthetics could deliver the protection needed to withstand supersonic flight.

Born To Fly
One person who’d come to understand this firsthand was AI Amatuzio, Lieutenant Colonel and squadron commander in the Minnesota Air National Guard. Stationed in Duluth, Minn., Amatuzio had experienced the benefits of synthetic lubricants in his squadron’s jet aircraft.
Amatuzio had taken an interest in aviation from a young age as he watched the Sikorsky mail plane fly over his neighborhood on its way to Lake Superior’s St. Louis Bay. At 12, a short ride in a Piper Cub* cemented his love of aircraft.
In 1942, Amatuzio answered America’s call during WWII. He attended Naval Air Corps training until the Navy closed the program. After the war and eager to again pursue his dream of flying, Amatuzio joined the Air Force. He helped usher in the era’s new jet-aircraft technology by flying the F80 Shooting Star.
“If it works that well in aircraft … ?”
Seeing synthetic oil in action, Amatuzio wondered why it wasn’t used in automobile engines. He reasoned that the same performance benefits could be applied to the vehicles and equipment people depended on every day for work and fun.
When Amatuzio began researching synthetic oil in the 1960s, motor oil quality was poor and engines didn’t last long. Then-modern oils were susceptible to breakdown in high heat and contributed greatly to hard-starting in cold weather. Oil industry giants thought conventional oils were good enough and thought synthetic oil was unnecessary for passenger cars.
Amatuzio undertook an intense period of research and development. He experimented with various formulations. He studied chemistry and learned about additives. In 1966, Amatuzio had formulated his first synthetic motor oil. To test his formulation, he asked one of his pilots to use it in his brand-new 1966 Ford* station wagon.
Throughout the late 1960s, Amatuzio continued to develop and sell synthetic oils under a variety of names. By 1968, he was commercially selling his synthetic motor oil. He incorporated “Life-Lube, Inc.” on May 23, 1969 and continued to commercially sell various synthetic motor oil formulations.
By 1970, Amatuzio had settled on a single formulation and had renamed his company “AMZOIL” – an amalgamation of his name and “oil” – which he’d later change to “AMSOIL.”
Still serving in the Air National Guard, Amatuzio ran his company in his spare time, working from his basement and warehousing product in his garage.
AMSOIL Synthetic Motor Oil became the world’s first synthetic motor oil to meet API service requirements. It outperformed conventional petroleum motor oils on all counts, heralding a new age in lubricant performance and engine life.
His financial resources, however, didn’t match his energy, and he nearly bankrupted himself leading his fledgling company. Since no one believed in his idea, no one would lend him money. And few motorists were willing to pay for synthetic motor oil no matter how profound its performance benefits since it cost several times more than conventional motor oil.

The World’s First API-Qualified Synthetic Motor Oil
The omission of two important sets of letters on each can of oil also slowed sales: API and SAE. To earn the trust of motorists, AMSOIL Synthetic Motor Oil needed to meet the industry performance standards established by the American Petroleum Institute (API) and Society of Automotive Engineers (SAE).
In 1972, Amatuzio sent AMSOIL Synthetic Motor Oil to an
accredited third-party laboratory, where it was subjected to a battery of
industry tests.
The
result? AMSOIL Synthetic Motor Oil became the world’s first synthetic motor oil
to meet API service requirements. It outperformed conventional petroleum motor
oils on all counts, heralding a new age in lubricant performance and engine
life.
Resistant To Change
From day one, synthetic motor oil was foreign to the Big Oil companies and automotive manufacturers of the time. AMSOIL Synthetic Motor Oil was guaranteed for 25,000 miles/one year, and other oil companies viewed such performance as detrimental to continuous sales. They didn’t want synthetic oil, nor did they believe cars needed it. They were satisfied with the status quo, and Amatuzio was ridiculed for peddling his “fake oil.”
Eventually Mobil: the king of the oil industry, acquiesced and introduced its synthetic oil in 1974. The automotive industry also slowly warmed up to synthetic motor oil’s benefits. Largely in response to the energy crisis of the late 1970s, automakers began to introduce smaller, hotter-running, highly efficient engines that delivered more power and greater fuel economy than their predecessors. Synthetic lubricants gained popularity thanks to their ability to withstand the intense heat, pressure and stress of modern high-tech engines. Chevron* introduced a synthetic oil in 1990, while Valvoline* followed suit in 1992. Eventually, every major oil manufacturer introduced a synthetic oil of its own.
The same companies that had deemed conventional oil “good enough” a few decades earlier soon embraced synthetic lubricants as an enabler of higher levels of performance not thought of years before.
Hall Of Fame Induction
The seismic shift in thinking, however, started three decades earlier when Amatuzio wondered why we weren’t using synthetic oil in our cars and trucks and set to work changing the status quo. His contributions to the synthetic lubricant industry were validated in 1994 when he was inducted into the Lubricants World Hall of Fame, an honor that confirmed his status as a pioneer and thought-leader. His company had grown into a world leader in synthetics and had since introduced several other industry firsts to the market, including the first synthetic gear lube for automotive use, the first synthetic diesel oil and the first 100:1 synthetic two-stroke oil.
Today, more than 50 years after Amatuzio began commercially selling synthetic motor oil, AMSOIL INC. has solidified its status as the premier manufacturer of synthetic lubricants in the world. AMSOIL products are available in more than 60 countries, lubricate approximately half the wind turbines in North America and represent the only choice of millions of discerning enthusiasts across the U.S. and Canada.

*All trademarked names and images are the property of their respective owners and may be registered marks in some countries. No affiliation or endorsement claim, express or implied, is made by their use. All products advertised here are developed by AMSOIL for the use in the application shown.
Reproduced With The Permission Of AMSOIL INC. All Rights Reserved.
